New fluorescent probes for explosive TATP

TATP
- Peroxide-based explosives such as Triacetonetriperoxide (TATP) are arguably one of the greatest terrorism threats faced today
- Nicknamed “The Mother of Satan,” TATP is a sensitive and easily-produced high explosive used traditionally by terrorist organizations
- TATP is a white crystalline powder that can resemble sugar or methamphetamine. Extreme caution must be taken to safely identify any white powders in a suspected lab or improvised explosive device (IED
- TATP is inherently very volatile, has the potential to sublime from a solid to a vapor, and can be detonated by the slightest friction from handling it
Solvent-Free Off-On detection of TATP with Fluorogenic Materials (M5-1b Membrane)
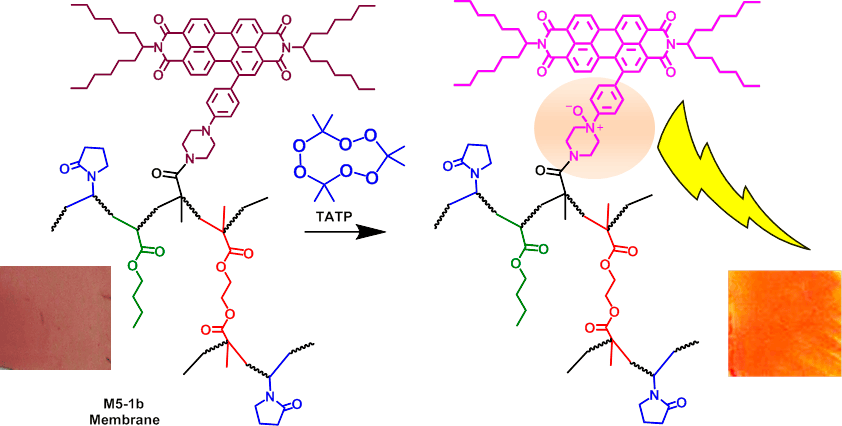
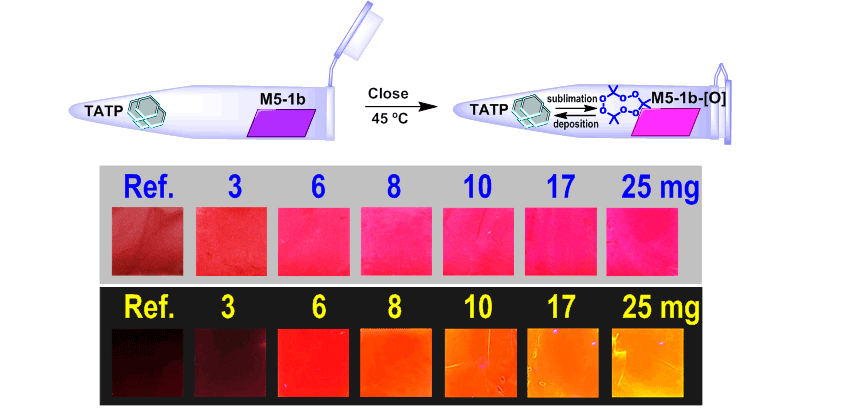
The qualitative effect of exposing the M5-1b membrane to increasing amounts of solvent-free TATP (A) under white light and (B) under UV light (366 nm)
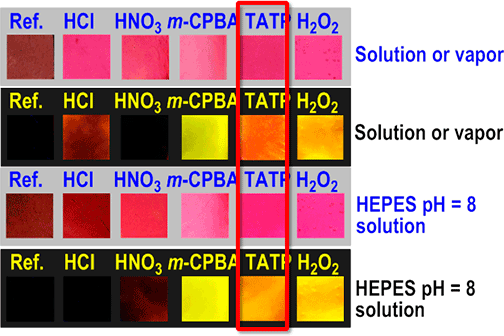
Color and fluorescence changes of membrane M5-1b with some acids and oxidants
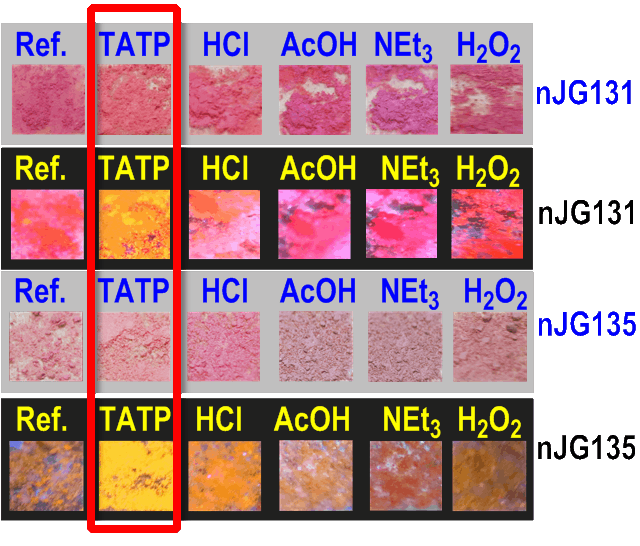
First row (all under white light):
- Ref.: pristine M5-1b
- HCl: M5-1b in the presence of the vapors of a drop of HCl 35%
- HNO3: M5-1b in the presence of the vapors of a drop of HNO3 65%
- m-CPBA: addition of MCPB acid (50 mM in CHCl3, 500 µL)
- TATP: M5-1b in the presence of 25 mg of solid TATP for 1 hours at 50 ºC in a Teflon vial
- H2O2: addition of H2O2 30% (500 µL)
Second row: same experimental conditions than in the first row, but under a UV lamp, 366 nm.
Third row (all under white light):
- Ref.: pristine M5-1b
- HCl: M5-1b in the presence of the vapors of a drop of HCl 35% and then addition of HEPES pH = 8, 500µL
- HNO3: M5-1b in the presence of the vapors of a drop of HNO3 65% and then addition of HEPES pH = 8, 500µL
- m-CPBA: addition of MCPB acid (50 mM in CHCl3, 500 µL)
- TATP: M5-1b in the presence of 25 mg of solid TATP for 1 hour at 50 ºC in a Teflon vial and then addition of HEPES pH = 8, 500 µL
- H2O2: addition of H2O2 30% (500 µL)
Fourth row: same experimental conditions than in the third row, but under a UV lamp, 366 nm
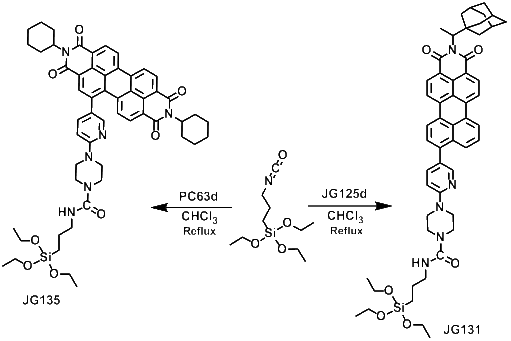
Off-On Fluorogenic Detection of Vapour Traces of TATP (nJG131 and nJG135)
Synthesis of nJG131 and nJG135
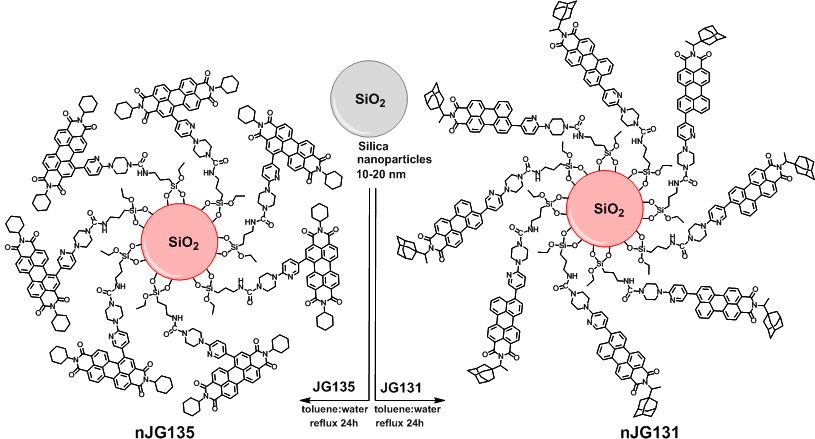
Detection of TATP in the gas phase by the colour and fluorescence changes of modified silica nanoparticles
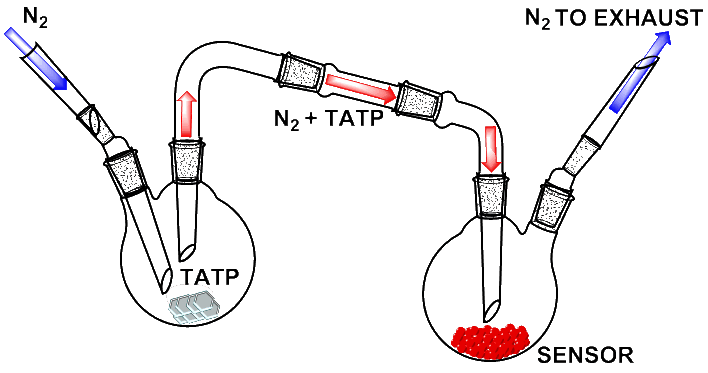
Glassware system design for the detection of TATP in the gas phase with nJG131/pJG131 and nJG135/pJG135
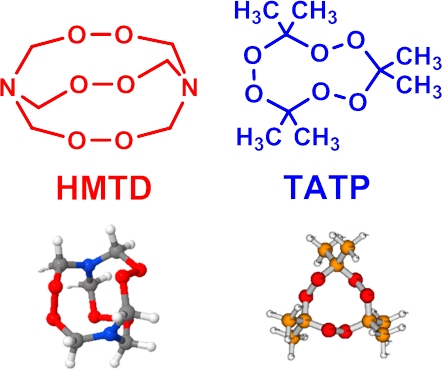
Hexamethylene triperoxide diamine (HMTD) versus TATP
- Despite no longer being used in any official application, HMTD remains a fairly popular home-made explosive and has been used in a large number of suicide bombings throughout the world
- HMTD can be difficult to measure due in part to its instability combined with its very low vapor pressure as determined from empirical observations
- Fast and sensitive detection of the intact HMTD for unequivocal identification is required
- Because of its difficulty, HMTD detection has been much less studied than TATP detection
- Currently, there are no methods of HMTD detection by fluorescent probes. Methods to discriminate between HMTD and TATP by fluorescence can be developed
Checking of perylene and naphthalene monoimides as chemical probes for TATP and HMTD
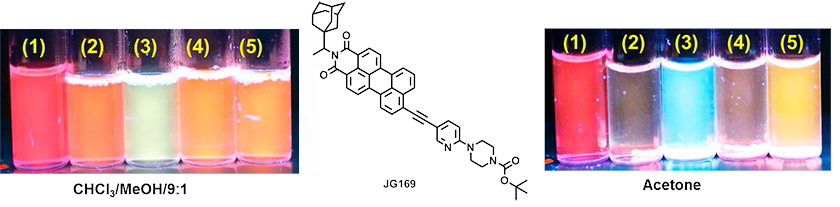
Checking the sensitivity in solution of a perylenemonoimide derivative (2×10-5M)
(1) Ref, (2) Amberlite IR-120(H), (3) Amberlite + m-CPBA, (4) Amberlite + TATP, (5) Amberlite + HMTD
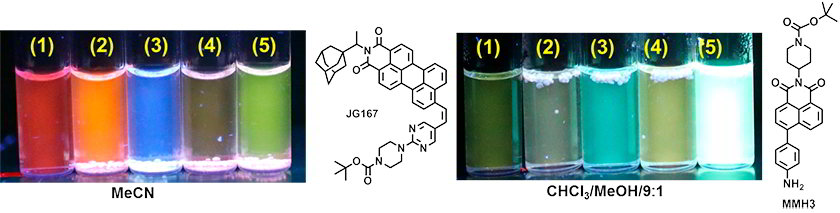
Checking the sensitivity in solution of perylene or naphthalene monoimide derivatives (2×10-5M) (1) Ref, (2) Amberlite IR-120(H), (3) Amberlite + m-CPBA, (4) Amberlite + TATP, (5) Amberlite + HMTD
Detection of trace explosives under ambient conditions using Multiphoton Electron Extraction Spectroscopy (MEES)
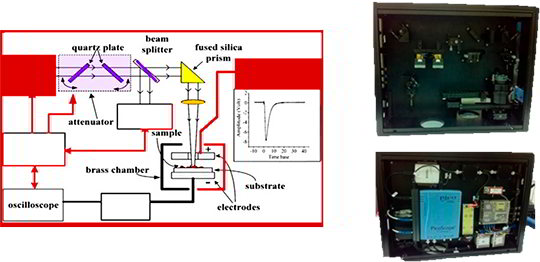
The MEES experimental setup scheme (left) and its packing (right)
- Direct induction of multiphoton processes
- Method provides detailed spectral features, as well as excellent sensitivity
- Potential application of MEES to analysis of trace improvised and traditional explosives under field conditions
- The optical technique named Multiphoton Electron Extraction Spectroscopy (MEES) allows for direct analysis of solid surfaces under ambient conditions
- The method is fast (a few seconds) and does not require pre-treatment of the examined materials
- When scanning the surface, the method provides chemical imaging & 2D chemical morphology
- The analytical LODs are in the sub-pmol range
- The method has been tested for practical security
- The method is a good candidate for security applications
